PRODUCT QUALITY MANAGEMENT
Quality reliably crafted.
Every part we produce meets the highest standards of precision and reliability.
Measurement & Inspection
First Article Inspection (FAI)
For our plastics injection molding services, FAI involves detailed measurements and evaluations of the initial part against design specifications and tolerances. This step helps identify discrepancies early, allowing for adjustments before full-scale production begins. By choosing Aprios, you can be confident that we will catch issues at the outset, ensuring that the final production runs smoothly and meets your quality standards from the very first part.
Production Part Approval Process (PPAP)
For many industries, a strong set of documentation provides additional assurance that the production process for your product is robust. At Aprios, our experience with PPAP includes thorough documentation such as PFMEA, Control Plans, and Validation. PPAP helps confirm each production run meets your standards for quality and stays consistent from batch to batch. It’s part of our structured approach to validating that manufacturing processes stay capable, repeatable, and under control from start to finish.
What Are Product Quality Management Services?
Validation Protocols
Process Validation: IQ/OQ/PQ
By maintaining comprehensive Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) processes, we ensure that our equipment and processes are thoroughly vetted and capable of meeting your production needs. The goal is simple: stable runs, fewer problems, and quality that holds up from start to finish.
IQ is the process of ensuring that equipment is properly installed according to manufacturer specifications and industry standards. For our plastics injection molding and additive manufacturing services, IQ involves verifying that major equipment, such as Injection Molding Machines and Sonic Welders, are set up correctly and safely. This step prevents problems caused by incorrect installation all while keeping production running safely from the start. With each setup verified, you gain confidence that our equipment runs as intended, reducing downtime and rework before production begins.
The purpose of OQ is to ensure that key process parameters produce repeatable parts within the process limits. OQ generally consists of production runs at the low and high process window limits, with a statistically relevant sampling of components. OQ testing confirms the process stays stable and makes parts that meet spec across different runs. It’s how we confirm real-world capability before scaling up, so you know the process is locked in and ready for dependable production.
PQ is the final step that involves validating that the process and equipment consistently produce products that meet all predefined criteria under real-world conditions. For our company, this means running the equipment under normal production conditions at nominal process parameters to ensure that every part we produce meets your quality standards. PQ shows that every step of production performs as expected in real conditions and is the final confirmation that we can maintain steady output, minimize variation, and deliver high-quality parts across every batch.
Advanced Equipment and Certifications
We utilize advanced measurement and inspection equipment to maintain high standards of quality.
Coordinate Measuring Machines (CMMs)
- Two Zeiss Contura CMMs, programmable with touch probe-based scanning for comparison to CAD models, ensure precise measurements.
Microscopes and Vision Systems
- MicroVu Vertex 200 and MicroVu Vertex 420 vision systems provide detailed inspection capabilities for complex parts.
Certifications
Our commitment to quality is demonstrated by our compliance with industry standards and certifications, including:
These certifications highlight our dedication to maintaining rigorous quality processes and standards across all operations. At Aprios, we built our quality system around consistency so we can deliver the same reliable results every time while keeping your production efficient and predictable.
These certifications highlight our dedication to maintaining rigorous quality standards across all our processes and services.
At Aprios, we strive to exceed your expectations by delivering reliable and efficient manufacturing solutions that enhance your supply chain and ensure the highest level of customer satisfaction.




Frequently Asked Questions
-
How do you ensure that your products meet customer expectations?
We follow a clear quality system built around inspection, testing, and documentation and every job runs through defined checks to make sure the final parts match your drawings and performance requirements. Feel free to view our certifications on our Certifications page.
-
What certifications does your quality management system hold?
Our quality system is certified to ISO 9001 and ISO 13485 — certifications that confirm our processes meet the standards required for both industrial and medical manufacturing. Details of which are listed on our Certifications page.
-
What happens if a quality issue comes up?
If something’s off, we stop production to figure out why, track the cause, make the fix, and document what changed. The goal is simple — solve it fast and keep it from happening again.
-
Do you provide quality documentation for your products?
Yes. Every order includes full inspection records, material certs, and compliance paperwork so you have traceability from start to finish.
-
How do you ensure compliance with industry standards?
We keep our quality system current with the standards and regulations we work under. When requirements change, we immediately will update our procedures. Scheduled audits and regular training confirm we’re compliant on every job.
-
Do you have a place where customers can view your certifications?
The following certifications back our North and West facilities located in Minneapolis, MN and Vista, CA:
ISO 9001:2015 - North
ISO 13485: 2016 - North
ISO 9001:2015 - West
ISO 13485: 2016 - West -
What do you mean by product auality management?
Product Quality Management is how we make sure every part meets print and customer expectations. It covers inspection, validation, and recordkeeping so work stays consistent, compliant, and traceable from start to finish.
-
How Does Product Quality Management Service Work?
Our team oversees each step of production, from setup through final inspection, to make sure parts meet spec and keep records as we go and watch how the job runs. If anything drifts, we make small changes right then to keep the parts coming out right.
Need Assurance on
Product Quality?



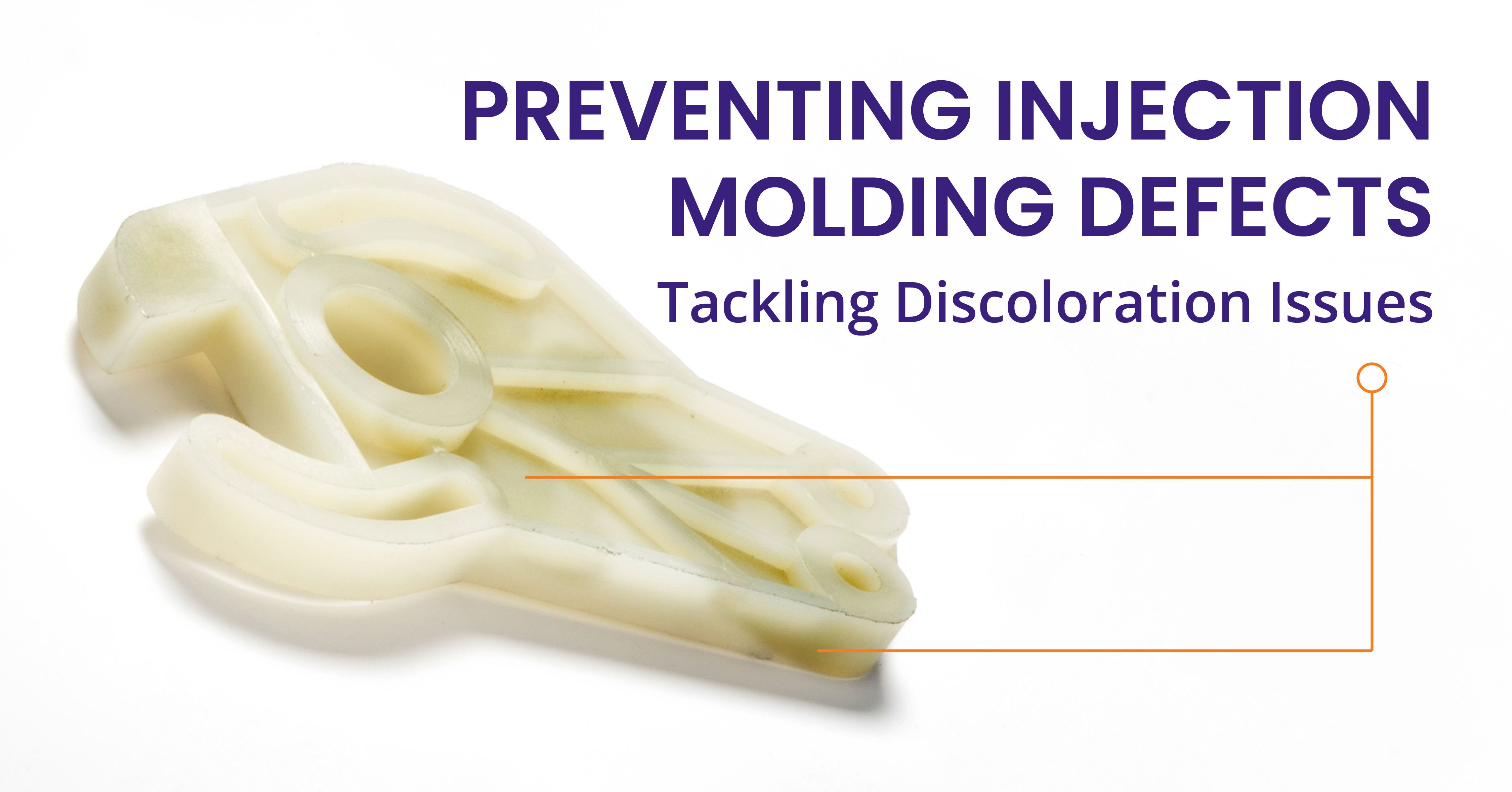





Quality in Injection Molding: Ensuring Consistency & Confidence
A behind-the-scenes look at how Aprios delivers precision, reliability, and compliance in every molded part.
WHAT'S INSIDE:
- Design of Experiment (DOE) for process optimization
- Process Validation (IQ, OQ, PQ) to ensure repeatability
- Gauge R&R for measurement accuracy
- First Article Inspection (FAI) & PPAP for production approval
- Proven strategies to minimize risk and maintain consistency